Answer:
Step-by-step explanation:
From the given information:
The equation for the reaction can be represented as:
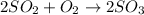
The I.C.E table can be represented as:
2SO₂ O₂ 2SO₃
Initial: 14 2.6 0
Change: -2x -x +2x
Equilibrium: 14 - 2x 2.6 - x 2x
However, Since the amount of sulfur trioxide gas to be 1.6 mol.
SO₃ = 2x,
then x = 1.6/2
x = 0.8 mol
For 2SO₂; we have 14 - 2x
= 14 - 2(0.8)
= 14 - 1.6
= 12.4 mol
For O₂; we have 2.6 - x
= 2.6 - 1.6
= 1.0 mol
Thus;
[SO₂] = moles / volume = ( 12.4/50) = 0.248 M ,
[O₂] = 1/50 = 0.02 M ,
[SO₃] = 1.6/50 = 0.032 M
Kc = [SO₃]² / [SO₂]² [O₂]
= ( 0.032²) / ( 0.248² x 0.02)
= 0.8325
Recall that; the equilibrium constant for the reaction
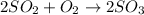 = 0.8325;
= 0.8325;
If we want to find:
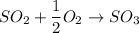
Then:
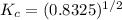
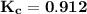
Since no temperature is given to use in the question, it will be impossible to find the final temperature of the mixture.