Answer:
6.71dm³
Step-by-step explanation:
Given parameters:
Initial Volume of hydrogen gas = 8.98dm³
Initial Temperature = 38.8°C; in Kelvin = 38.8 + 273 = 311.8K
Final temperature = -39.9°C; in Kelvin = -39.9 + 273 = 233.1K
Unknown:
Final volume = ?
Solution:
To solve this problem, we apply the Charles's law. It states at;
"The volume of a fixed mass (mole) of a gas varies directly as its absolute temperature if the pressure is constant".
Mathematically;
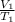 =
=
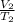
V and T are temperature values
1 and 2 are the initial and final states
Insert the parameters and solve for V₂
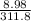 =
=
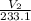
V₂ x 311.8 = 8.98 x 233.1
V₂ = 6.71dm³