Answer:
92.75%
Step-by-step explanation:
The overall chemical equation for the reaction in the preparation of alum from the aluminium can be expressed as:
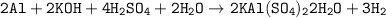
From above; we will see that 2 moles of Aluminium react with sulphuric acid and water to produce 2 moles o aluminium alum.
Therefore, the theoretical yield can be determined as:
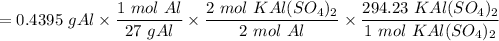
= 4.789g of
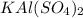
To find the percent yield, we need to divide the actual yield by the theoretical yield and then multiply it with 100.
∴
percent yield = ( mass of alum(g)/theoretical yield(g) ) × 100
percent yield = ( 4.789g / 5.1629g ) × 100%
percent yield = 0.9275 × 100%
percent yield = 92.75%
Thus, the percent yield of the experiment 92.75%