Answer:
Ka = 3.50x10⁻⁴
Step-by-step explanation:
First, we need to convert the unit of 3.60 g/L to mol/L:
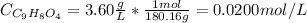
The reaction dissociation of aspirin in water is:
C₉H₈O₄ + H₂O ⇄ C₉H₇O₄⁻ + H₃O⁺
0.02 - x x x
The constant of the above reaction is:
![Ka = ([C_(9)H_(7)O_(4)^(-)][H_(3)O^(+)])/([C_(9)H_(8)O_(4)])](https://img.qammunity.org/2021/formulas/chemistry/college/gnjivddv15sazr78l775oz01hh24pyk8v8.png)
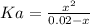
To find Ka we need to find the value of x. We know that pH = 2.6 so:
![pH = -log[H_(3)O^(+)]](https://img.qammunity.org/2021/formulas/chemistry/college/26pnglinrxk4r5gz37s10x7noz3afcooah.png)
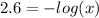
![x = 2.51 \cdot 10^(-3) M = [H_(3)O^(+)] = [C_(9)H_(7)O_(4)^(-)]](https://img.qammunity.org/2021/formulas/chemistry/college/8p2v7o5kh9q4yh8dcv1msjcq37z438abf1.png)
Now, the concentration of C₉H₈O₄ is:
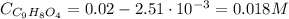
Finally, Ka is:
![Ka = ([C_(9)H_(7)O_(4)^(-)][H_(3)O^(+)])/([C_(9)H_(8)O_(4)]) = ((2.51 \cdot 10^(-3))^(2))/(0.018) = 3.50 \cdot 10^(-4)](https://img.qammunity.org/2021/formulas/chemistry/college/i9y8xgx09fzq55gcjeb8b9gkxnpp2zjlky.png)
Therefore, the Ka of aspirin is 3.50x10⁻⁴.
I hope it helps you!