The equation for the half life of a substance:
N(t) = N₀ (
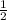 )
)
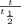
- N(t) = substance remaining
- N₀ = initial amount of substance
- t = time passed
- t
 = half life
= half life
Plug in the information you know into the equation:
100 = 800 (
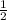 )
)
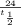
Now solve for
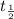 and you will get 8 hours as the half life
and you will get 8 hours as the half life