Answer:
300.9mL
Step-by-step explanation:
Given parameters:
V₁ = 280mL
T₁ = 22°C
T₂ = 44°C
Unknown:
V₂ = ?
Solution:
To solve this problem, we apply Charles's law;
it is mathematically expressed as;
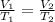
We need to convert the temperature to kelvin;
T₁ = 22°C = 22 + 273 = 295K
T₂ = 44°C = 44 + 273 = 317K
Input the parameters and solve;
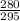 =
=
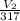
V₂ x 295 = 280 x 317
V₂ = 300.9mL