Answer:
4.52 x 10²⁴ atoms Cl
Step-by-step explanation:
A mole is a name that means a certain number like a dozen means 12. 1 mole of chlorine atoms is 6.022 x 10²³ chlorine atoms. The unit conversion is 6.022 x 10²³/mol.
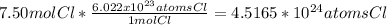
Round to 4.52 x 10²⁴ atoms Cl for the correct number significant figures.