Answer:
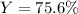
Step-by-step explanation:
Hello.
In this case, since no information about the reacting hydrogen is given, we can assume that it completely react with the 28.0 g of acetylene to yield ethane. In such a way, via the 1:1 mole ratio between acetylene (molar mass = 26 g/mol) and ethane (molar mass = 30 g/mol), we compute the yielded grams, or the theoretical yield of ethane as shown below:
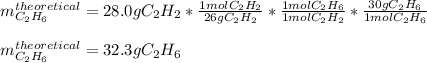
Hence, by knowing that the percent yield is computed via the actual yield (24.5 g) over the theoretical yield, we obtain:
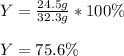
Best regards.