Answer:
The answer is 0.78 mol/dm³
Step-by-step explanation:
In order to calculate the molarity of the solution we use the formula
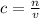
where
c is the molarity
n is the number of moles
v is the volume in dm³
From the question
n = 7 mol
v = 9 L = 9 dm³
We have

We have the final answer as
0.78 mol/dm³
Hope this helps you