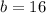Answer:
The other leg is 16 cm long.
Step-by-step explanation:
Use the Pythagorean theorem:
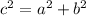 ,
,
where:
 is the hypotenuse, and
is the hypotenuse, and
 and
and
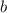 are the other two sides (legs).
are the other two sides (legs).
Let
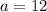
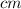
Rearrange the equation to isolate
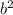 . Plug in the values for
. Plug in the values for
 and
and
 , and solve.
, and solve.
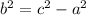
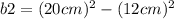
Simplify.
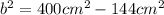
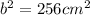
Take the square root of both sides.
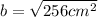
Simplify.