Answer:
multiply them
Explanation:
Units work in algebraic equations the same way that variables do. They can be multiplied and divided as though they were variables.
For units conversion, we want to change the units without changing the value of the quantity. We do that by multiplying by a unit conversion factor that has a numerator equal to its denominator.
__
We know ...
1 cup = 8 fl oz
2 cups = 1 pint
These relations can be written as the conversion factors ...
(8 floz)/(1 cup) and (2 cups)/(1 pint)
Multiplying these together, the units of cups cancel, leaving fluid ounces per pint.
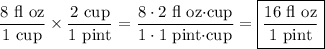
_____
Additional comment
The concept of "like terms" applies to expressions involving units as well as expressions involving variables. Terms can only be added or subtracted if they have the same units.