Answer:
Q = 65 kJ
Step-by-step explanation:
Given that,
Mass, m = 580 g
Initial temperature,
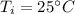
Final temperature,
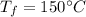
The specific heat capacity for aluminum is 0.897 J/g°C
We need to find the heat added to the aluminium pan so that its heat raised to a temperature from 25°C to 150°C. It is given by :
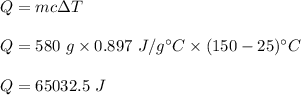
or
Q = 65.03 kJ
or
Q = 65 kJ
Hence, the heat added to the pan is 65 kJ.