Zinc's most abundant isotope : Zinc-65
Further explanation
Isotopes are atoms whose no-atom has the same number of protons while still having a different number of neutrons.
So Isotopes are elements that have the same Atomic Number (Proton)
Atomic mass is the average atomic mass of all its isotopes
In determining the mass of an atom, as a standard is the mass of 1 carbon-12 atom whose mass is 12 amu
Mass atom X = mass isotope 1 . % + mass isotope 2.%
To decide zinc's most abundant isotope, then choose the closest mass number
or we can check the difference with the average mass number, if the value is the smallest, then that isotope has the largest abundant
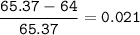
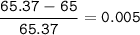
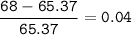
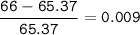
The closest = Zinc-65(the smallest)