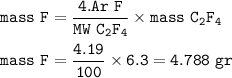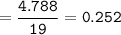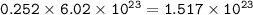Fluorine atoms = 1.517 x 10²³
Further explanation
The mole is the number of particles contained in a substance
1 mol = 6.02.10²³
Moles can also be determined from the amount of substance mass and its molar mass

Proust stated the Comparative Law that compounds are formed from elements with the same Mass Comparison so that the compound has a fixed composition of elements