Answer:
Answers in solutions.
Step-by-step explanation:
Question 6:
The density of gold is 19.3 g/cm³
The density of silver is 10.5 g/cm³
- The density of the substance in Crown A;
Density = mass ÷ volume =
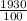 = 19.3 g/cm³
= 19.3 g/cm³
Since the density of gold, given, is 19.3 g/cm³ and the density of the substance in Crown A has a density of 19.3 g/cm³ , then that substance must be gold.
- The density of the substance in Crown B;
Density = mass ÷ volume = 1930 ÷ 184 = 10.48913043 g/cm³ ≈ 10.5 g/cm³ (answer rounded up to one decimal place)
Since the density of the substance in Crown B is approximately equal to 10.5 g/cm³ , then that substance is Silver.
- The density of substance in Crown C;
Density = mass ÷ volume = 1930g ÷ 150cm³ = 12.86666667 ≈ 12.9 cm³ (answer rounded up to one decimal place)
The density of the mixture:
For 2 cm³ of the mixture, its mass equal 19.3 g + 10.5 g = 29.8 g
∴ for 1 cm³ of the mixture, its mass equal to
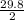 = 14.9 g
= 14.9 g
Hence the density of the mixture = 14.9 g/cm³ and is not equal to the density of the substance in Crown C.
* Crown C is not made up of a mixture of gold and silver.
Question 7:
- An empty masuring cylinder has a mass of 500 g.
- Water is poured into measuring cylinder until the liquid level is at the 100 cm³ mark.
- The total mass is now 850 g
The mass of water that occupied the 100 cm³ space of the container = total mass - mass of the empty container = 850 g - 500 g = 350 g
Density of the liquid (water) poured into the container = mass ÷ volume = 350 g ÷ 100 cm³ = 3.5g/cm³
Question 8:
A tank filled with water has a volume of 0.02 m³
(a) 1 liter = 0.001 m³
How many liters? = 0.02 m³ ?
Cross multiplying gives:
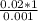 = 20 liters
= 20 liters
(b) 1 m³ = 1,000,000 cm³
0.02 m³ = how many cm³ ?
Cross-multiplying gives;
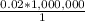 = 20,000 cm³
= 20,000 cm³
(c) 1 cm³ = 1 ml
∴ 0.02 m³ of the water = 20,000 cm³ = 20,000 ml
Question 9:
Caliper (a) measurement = 3.2 cm
Caliper (b) measurement = 3 cm
Question 10:
- A stone is gently and completely immersed in a liquid of density 1.0 g/cm³
- in a displacement can
- The mass of liquid which overflow is 20 g
The mass of the liquid which overflow = mass of the stone = 20 g
1 gram of the liquid occupies 1 cm³ of space.
20 g of the liquid will occupy;
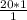 = 20 cm³
= 20 cm³
(a) Since the volume of the water displaced is equal to the volume of the stone.
∴ The volume of the stone = 20 cm³
(b) Mass = density × volume
Density of the stone = 5.0 g/cm³
Volume of the stone = 20 cm³
Mass of the stone = 5 g/cm³ × 20 cm³ = 100 g