Answer:
The mass of aluminum is 124.72 g
Step-by-step explanation:
Calorimetry is the measurement and calculation of the amounts of heat exchanged by a body or a system.
Sensible heat is the amount of energy that causes a change in the temperature of a substance. In other words, sensible heat is that heat that causes the temperature of an object to vary without affecting its molecular structure and therefore its physical state. Its mathematical expression is:
Q = c * m * ΔT
where Q is the heat exchanged by a body of mass m, constituted by a substance of specific heat c and where ΔT is the variation in temperature.
In this case:
- Q= 900 J
- c= 0.902
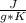
- m= ?
- ΔT= Tfinal - Tinitial= 38 °C - 30°C= 8°C as it is a temperature difference, it is indistinct if the temperature is expressed in ° C or ° K because the difference will be the same. So: ΔT= 8°C= 8 °K
Replacing:
900 J= 0.902
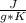 *m* 8 °K
*m* 8 °K
900 J= 7.216
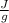 *m
*m
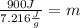
124.72 g=m
The mass of aluminum is 124.72 g