Answer:
- Krypton.
- Silicon.
Step-by-step explanation:
Hello.
In this case, since the first element's last term in the electron configuration is 4p⁶, by looking at the fourth period, the only element having sic 4p electrons is Kr as its electron configuration is:
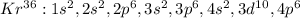
In the second case, the element of the third period having two 3p electrons or
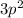 the last term in its electron configuration is silicon as shown below:
the last term in its electron configuration is silicon as shown below:
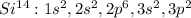
Best regards.