Answer:
A) During this procedure ( hypoventilation ) The CO2 in the arterial blood vessels and the lungs increases and this drives the PH level in the system lower, and the equilibrium will shift to the right. this is because the Blood-PH level is controlled by CO2 - bicarbonate buffer system
B) The blood PH may rise to 7.60 during Hyperventilation because the removal of CO2 from the lungs causes the increase in
 which is directly proportional to the increase in Blood PH levels
which is directly proportional to the increase in Blood PH levels
C) Hyper ventilation before a dash would be useful because it will remove excessive Hydrogen ions and and raise the Blood PH levels in preparedness of the production of acids like Lactic acid
Step-by-step explanation:
A) During this procedure ( hypoventilation ) The CO2 in the arterial blood vessels and the lungs increases and this drives the PH level in the system lower, and the equilibrium will shift to the right. this is because the Blood-PH level is controlled by CO2 - bicarbonate buffer system
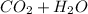 ⇄
⇄
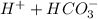
B) The blood PH may rise to 7.60 during Hyperventilation because the removal of CO2 from the lungs causes the increase in
 which is directly proportional to the increase in Blood PH levels
which is directly proportional to the increase in Blood PH levels
C) Hyper ventilation before a dash would be useful because it will remove excessive Hydrogen ions and and raise the Blood PH levels in preparedness of the production of acids like Lactic acid