Given parameters:
Chemical specie = LiClO₂
Unknown:
Percentage composition of each specie = ?
Solution:
The percentage composition involves knowing the percent of total mass of a compound is made up of each of the constituent elements or groups.
- Sum up the atomic masses and find the formula mass
- Determine percentage composition of each element
Formula mass of LiClO₂;
7 + 35.5 + 2(16) = 74.5g/mol
The percentage composition of each species are;
%Li =
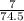 x 100 = 9.4%
x 100 = 9.4%
%Cl =
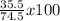 = 47.7%
= 47.7%
%O₂ =
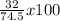 = 43.0%
= 43.0%