Answer:
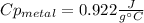
Step-by-step explanation:
Hello.
In this problem we must realize that there is heat flow that moves from the hot metal object and the hot water to the cold water and the cold aluminum cup, which allows us to write:
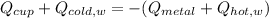
Which means that the heat lost be the hot metal object and the hot water is gained by both the cold water and the cold aluminum cup, which can be written in terms of mass, specific heats and change in temperature towards the equilibrium temperature (35.9 °C):
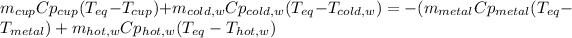
We need to solve for the specific heat of the metal as shown below:
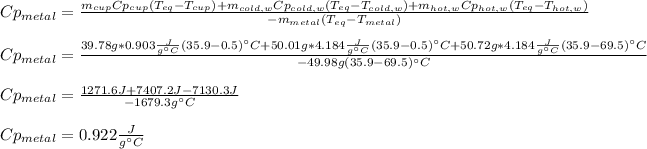 Best regards.
Best regards.