Answer:
(a). The specific volume at the final state is 0.25 m³/kg.
(b). The energy transfer by work is 50.4 kJ.
(c). The energy transfer by heat is -10.4 kJ.
Negative sign shows the direction of heat
Step-by-step explanation:
Given that,
Weight of carbon monoxide = 4 kg
Volume of tank = 1 m³
Constant rate of 14W for 1 hour
The specific internal energy of the carbon monoxide increases by 10 kJ/kg.
(a). We need to calculate the specific volume at the final state
Using formula of specific volume
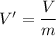
Put the value into the formula
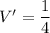
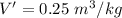
(b). We need to calculate the energy transfer by work
Using formula of work
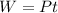
Where, P = power
t = time
Put the value into the formula
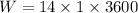
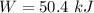
(c). The energy transfer by heat transfer, and the direction of the heat transfer
We need to calculate the
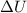
Using formula of internal energy
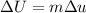
Put the value into the formula
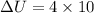
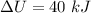
We need to calculate the energy transfer by heat
Using formula of energy transfer
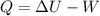
Put the value into the formula
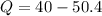
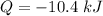
Negative sign shows the direction of heat that is removed from CO.
Hence, (a). The specific volume at the final state is 0.25 m³/kg.
(b). The energy transfer by work is 50.4 kJ.
(c). The energy transfer by heat is -10.4 kJ.
Negative sign shows the direction of heat.