Answer:
6.77 · 10²³ molecules H₂O
Step-by-step explanation:
Step 1: Define
2H₂ (g) + O₂ (g) → 2H₂O (g)
0.562 mol O₂
Step 2: Use Stoichiometry
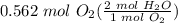 = 1.124 mol H₂O
= 1.124 mol H₂O
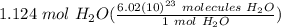 = 6.76648 · 10²³ molecules H₂O
= 6.76648 · 10²³ molecules H₂O
Step 3: Simplify
We have 3 sig figs.
6.76648 · 10²³ molecules H₂O ≈ 6.77 · 10²³ molecules H₂O