Answer:
a)
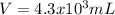
b)
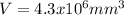
c)
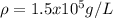
Step-by-step explanation:
Hello,
a) In this case, the given height in cm is:
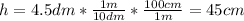
And the radius in cm is:
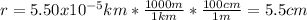
Thus, the volume in cubic centimeters which is also equal in mL (1cm³=mL) is:
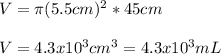
b) In this case, the given height in mm is:
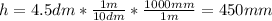
And the radius in mm is:
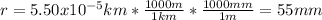
Thus, the volume in cubic millimeters is:
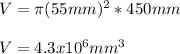
c) Finally, since 1000 mL equal 1 L, the required density in g/L turns out:
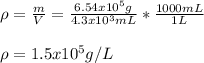
Best regards.