Answer:
d = 16.96 g/mL
Step-by-step explanation:
Given that,
Initial water level in graduated cylinder is 47 mL and final water level is 48.35 mL.
The weight of the neclace is 22.89 g.\
We need to find the volume of the necklace. We know that, density equals mass per unit volume. So,
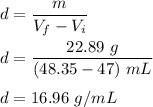 '
'
Hence, the density of Terry’s necklace is 16.96 g/mL.