Given :
A balanced chemical equation :
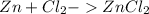
To Find :
How many grams of zinc are needed to produce 12 grams of zinc chloride.
Solution :
Moles of
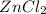 ,
,
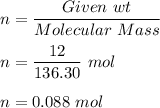
Now, by balanced chemical equation we can say that 1 mol of Zn produce
1 mol of
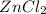 .
.
So, 0.088 mol of Zn is required to produced 0.088 mol of
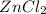 .
.
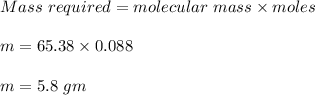
Therefore, 5.8 grams of zinc is required.
Hence, this is the required solution.