Answer:
The answer is
84.9 kPa
Step-by-step explanation:
Using Boyle's law to find the final pressure
That's
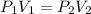
where
P1 is the initial pressure
P2 is the final pressure
V1 is the initial volume
V2 is the final volume
Since we are finding the final pressure
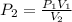
From the question
P1 = 115 kPa
V1 = 480 mL
V2 = 650 ml
So we have
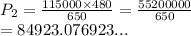
We have the final answer as
84.9 kPa
Hope this helps you