Answer:

Step-by-step explanation:
Hello
In this case, by considering the Raoult's law we can write:
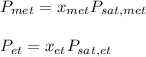
Whereas the purpose is to compute the pressures of both methanol and ethanol (pressures above the solution), thus, the first step is to compute the molar fractions:
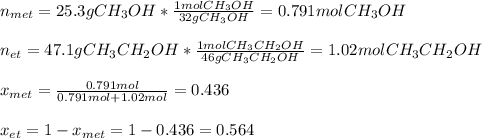
Therefore, the pressures turns out:
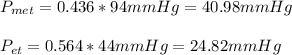
Best regards.