Answer:
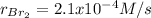
Step-by-step explanation:
Hello,
In this case, for the reaction:
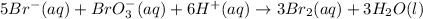
Thus, via the rate proportions between Br⁻ and Br₂ for which the stoichiometric coefficients are 5 and 3 respectively, we can write:
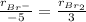
Hence, the rate of appearance of Br₂ turns out:
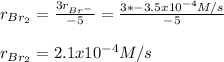
Take into account that the rate of disappearance is negative for reactants.
Best regards.