The balanced chemical equation is :
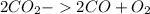
Moles of
 ,
,
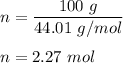
Now, by given chemical equation , we can see 2 mole of
 react with 1 mole of
react with 1 mole of
 .
.
So , 2.27 mole react with :
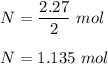
Mass of oxygen is :
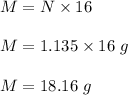
Therefore, mass of oxygen in grams produced is 18.16 g.
Hence, this is the required solution.