Given :
Compound A reacts with Compound B to form only one product, Compound C.
The usual percent yield of C in this reaction is 40%.
10.0 g of A are reacted with excess Compound B, and 6.4 g of Compound C
To Find :
The theoretical yield of C.
Solution :
We know, % yield is given by :
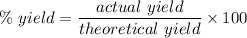
Putting given values , we get :

Therefore, theoretical yield of C is 16 g.
Hence, this is the required solution.