Answer:

Step-by-step explanation:
Volume can be found by dividing the mass by the density.
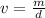
The mass is 0.64 kilograms and the density is 1.318 grams per cubic centimeter. Since the density is in grams, we must convert the mass to grams.
Convert the mass to grams.
- The mass is 0.64 kilograms
- 1 kilogram= 1,000 grams
- Multiply 0.64 and 1,000
- 0.64*1000=640
- 0.64 kg=640 g
Now we know the mass in grams and the volume, so we can substitute them into the formula.
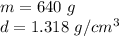
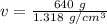
Divide. Note that the grams, or g will cancel.
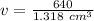
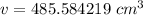
The volume is 485.584219 cubic centimeters.