Balanced chemical equation is :
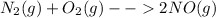
It is given that the equation is in equilibrium.
We need to find what will happen if we add more
 is added .
is added .
By Le Chatelier's principle :
Changing the concentration of a chemical will shift the equilibrium to the side that would counter that change in concentration.
It means production of the side where content is added will decrease and concentration on other side will increase .
So , more NO would form .
Therefore, option B. is correct.
Hence, this is the required solution.