Answer:
The absolute zero temperature T = - 269.91° C
Step-by-step explanation:
From the given information:
The plot of the volume of the experimental data against the celsius temperature can be expressed mathematically as:
V = mT + c
V = 0.0741 × T+c --- (1)
where;
m, which is the straight line of the slope = 0.0741
replacing the value of V to be = 20 L, T= 0.
Then:
20 = 0.0741 × 0 + c
20 = c
From equation (1), replacing the value of c with 20, we have:
V = 0.0741 × T+20
SO, to determine absolute zero temperature: we have the volume to be zero at absolute zero temperature, Thus:
0 = 0.0741 × T+20
0.0741 T + 20 = 0
0.0741 T = - 20
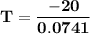
T = - 269.91° C