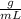Answer:
The unknown solid’s density is 0.071
Step-by-step explanation:
Density is defined as the property that matter, whether solid, liquid or gas, has to compress into a given space. In other words, density is a quantity that allows us to measure the amount of mass in a certain volume of a substance. Then, the expression for the calculation of density is the quotient between the mass of a body and the volume it occupies:
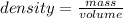
From this expression it can be deduced that density is inversely proportional to volume: the smaller the volume occupied by a given mass, the higher the density.
Archimedes' principle states that every body immersed in a fluid experiences a vertical and upward thrust equal to the weight of fluid dislodged. In other words, this principle says that the volume of the object is equal to the volume of the water displaced or displaced. So:
- mass= 2.5 grams
- volume=35 mL
Replacing in the definition of density:
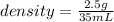
you get:
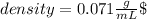
The unknown solid’s density is 0.071