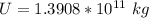Answer:
The value is
Step-by-step explanation:
From the question we are told that
mass of carbon dioxide produced by one gallon of gasoline is
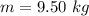
The number of cars is
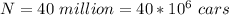
The distance covered by each car is
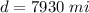
The rate is
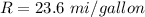
Generally the amount of gasoline used by one car is mathematically represented as
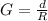
=>
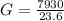
=>
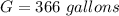
Generally the amount of gasoline used by N cars is
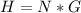
=>
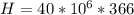
=>
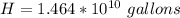
Generally the annual production of carbon dioxide is mathematically represented as
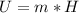
=>
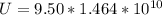
=>