Answer:
a.
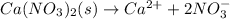
b.
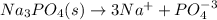
Step-by-step explanation:
Hello,
In this case, considering that the dissolution reaction of a solid in aqueous solutions involve the dissociation (separation) of both its cation and anion for the required salts we write:
a.
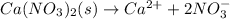
b.
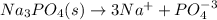
Thus, a two is required for nitrate anions in a. in order to balance it at the reactant and a three is required for sodium cations in b. in order to balance it as well.
Best regards.