Answer:
The shortest de Broglie wavelength for the electrons that are produced as photoelectrons is 0.81 nm
Step-by-step explanation:
Given;
wavelength of ultraviolet light, λ = 270 nm
work function of the metal, φ = 2.3 eV = 2.3 x 1.602 x 10⁻¹⁹ J = 3.685 x 10⁻¹⁹ J
The energy of the ultraviolet light is given by;
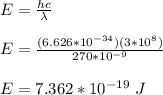
The energy of the incident light is related to kinetic energy of the electron and work function of the metal by the following equation;
E = φ + K.E
K.E = E - φ
K.E = (7.362 x 10⁻¹⁹ J) - (3.685 x 10⁻¹⁹ J )
K.E = 3.677 x 10⁻¹⁹ J
K.E = ¹/₂mv²
mv² = 2K.E
velocity of the electron is given by;
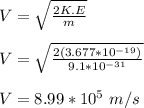
the shortest de Broglie wavelength for the electrons is given by;
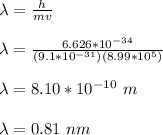
Therefore, the shortest de Broglie wavelength for the electrons that are produced as photoelectrons is 0.81 nm