Answer:
1) The power developed by the engine is 14705.7739 kW
2) The thermal efficiency is approximately 61.5%
Step-by-step explanation:
The given parameters are;
P₁ = 95 kPa
T₁ = 22°C
V₁ = 3.17 liters
The cutoff ratio = 2.5
Displacement volume = 3 liters
The number of times the cycle is executed per minute = 1000 times per minute
We have;
The displacement volume = V₁ - V₂ = 3 l
V₁ = 3.17 l
V₂ = 3 - 3.17 = 0.17 l
Compression ratio = V₁/V₂ = 3.17/0.17 ≈ 18.65
P₂/P₁ = P₂/(95 kPa) = (V₁/V₂)^(k) = 18.65^1.4
P₂ = (95×18.65^(1.4)) ≈ 5710.5 kPa
T₂/T₁ = (V₁/V₂)^(k - 1)
T₂/(295 K)= (18.65)^(1.4 - 1)
T₂ = 295 * (18.65)^(1.4 - 1) = 950.81 K
The cutoff ratio = V₃/V₂ = 2.5
T₃ = T₂ × V₃/V₂ = 2.5 * 950.81 K = 2377.025 K
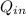 =
=
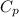 ×(T₃ - T₂) = 1.006 × (2377.025 - 950.81) = 1,434.77 kJ/kg
×(T₃ - T₂) = 1.006 × (2377.025 - 950.81) = 1,434.77 kJ/kg
T₄ = T₃ × (V₃/V₄)^(k-1) =
Therefore,
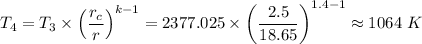
T₄ ≈ 1064 K
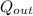 =
=
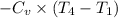
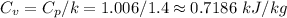
∴
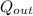 = 0.7186×(1064 - 295) = 552.6034 kJ/kg
= 0.7186×(1064 - 295) = 552.6034 kJ/kg
1) The net work =
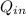 -
-
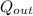 = 1,434.77 kJ/kg - 552.6034 kJ/kg ≈ 882.17 kJ/kg
= 1,434.77 kJ/kg - 552.6034 kJ/kg ≈ 882.17 kJ/kg
The number of cycle per minute = 1000 rpm
The number of cycle per minute = 1000 rpm/60 = 16.67 cycles per second
The power developed by the engine = The number of cycles per second × The net work of the engine
Therefore;
The power developed by the engine = 16.67 cycles/second × 882.17 kJ/kg
The power developed by the engine = 14705.7739 kW
2) Efficiency,
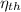 , is given as follows;
, is given as follows;
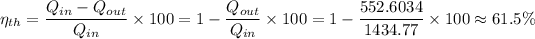
Therefore, the thermal efficiency ≈ 61.5%.