Answer:
2.0 mmole
Step-by-step explanation:
The computation fo the amount of mmol is shown below:
As we know that
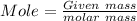
where,
Given mass is 0.2 gm
The Molar mass is 96.2g/mol
Now
put these values to the above formula
So,
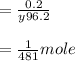
Mile mole is

= 2.079
= 2.0 mmole
As
mole = 1,000^-1 mili mole
We simply applied the above formula so that the amount of mmole could come