Answer:
please find the attached file.
Step-by-step explanation:
In the attachment file, we define the order of the ionization of the energy.
As its components are seen, their energy of ionization rises and falls further. Nitrogen and oxygen were detected as an anomaly. Nitrogen has more IE than oxygen because Nitrogen has the
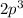 configuration to Is half-filled It's got bigger IE than B. As, be making the 2s2 configuration fully disc.
configuration to Is half-filled It's got bigger IE than B. As, be making the 2s2 configuration fully disc.