Given :
Nurse Antonio adds that 7 grams of NaCI to water to make 1 liter of solution.
To Find :
The molar concentration of the solution .
Solution :
Molecular mass of NaCl ,
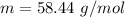 .
.
Now , number of moles is given by :
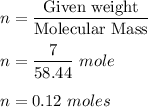
Molarity is given by :
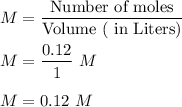
Hence , this is the required solution .