Answer: hello your question is incomplete below is the complete question
An ideal Otto Cycle has a compression ratio of 9.2 and uses air as the working fluid. At the beginning of the compression process, air is at 98kPa and 20C. The pressure is doubled during the constant volume heat addition process. Assuming constant specific heats, determine:the amount of heat transferred to the air
answer : 609.804 kj/kg
Step-by-step explanation:
Given data:
compression ratio (r)= 9.2
pressure given(p1) = 98 kPa
Initial temperature = 20 + 273 = 293 k
pressure during constant volume heat addition process = 2p1
note : specific heat at constant pressure and specific heat at constant volume varies with temperature
we use T = 300k because it is closest to T1 = 293 k
hence at T = 300 K ( ideal gas properties of air )
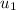 = 214.07 Kj/kg
= 214.07 Kj/kg
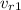 = 621.2
= 621.2
To get
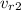 =
=
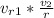 = 621.2 * 1 / 9.2 = 67.52
= 621.2 * 1 / 9.2 = 67.52
ALSO at
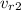 = 67.52 ( from ideal gas properties )
= 67.52 ( from ideal gas properties )
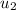 = 518.9 kj/kg
= 518.9 kj/kg
T2 = 708.32 k
next we apply the gas equation
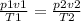
hence p2 = (9.2) *
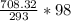 = 2179.59 kpa
= 2179.59 kpa
to determine T3 due to the constant volume heat addition
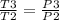
Hence T3 = p3/p2 * T2 = 2( 708.32 ) = 1416.64 k
At T3 = 1416.64 k ( from ideal gas properties )
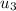 = 1128.704 kj/kg
= 1128.704 kj/kg
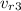 = 8.592
= 8.592
Determine the amount of heat transferred to the air
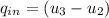
= ( 1128.704 - 518.9 )
= 609.804 kj/kg