Answer:
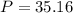
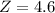
Step-by-step explanation:
Hello,
In this case, since the VdW equation is:
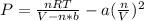
Since the moles are 10.0 moles, the temperature in K is 300.15 K and the volume is liters is also 4.860 L (1 dm³= 1L), the pressure exerted by the ethane is:
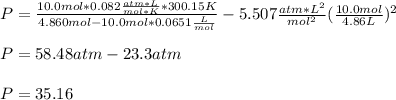
Thus the compression factor turns out:
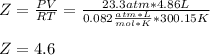
Regards.