Answer:
A. No precipitate is formed
Step-by-step explanation:
Hello,
In this case, since the reactions are:
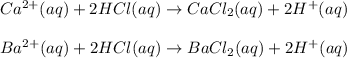
We evidence no formation of insoluble salts or precipitates since both calcium and barium chlorides are largely soluble in water due to the ionic bond which binds them, thus A. No precipitate is formed.
Regards.