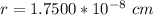Answer:
a
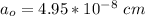
b
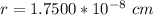
Step-by-step explanation:
From the question we are told that
The density of lead is
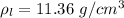
The atomic weight is
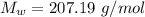
The Avogadro's number is
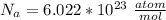
Generally for FCC the number of atom is n = 4
Generally the volume of a unit FCC cell is mathematically represented
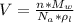
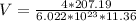
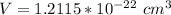
Generally the lattice parameter is mathematically represented as
![a_o = \sqrt[3]{V }](https://img.qammunity.org/2021/formulas/chemistry/college/sz65hvmyy8cam1b8kvvnz0nw62zdk96pcl.png)
![a_o = \sqrt[3]{1.211 5 *10^(-22) }](https://img.qammunity.org/2021/formulas/chemistry/college/3m353h6fu418xq0rhup0bj7ilyzc102k7m.png)
=>
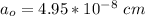
Generally the radius of the lead is mathematically represented as
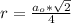
=>
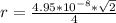
=>