Answer:
64 kg
Step-by-step explanation:
The computation of the number of kg need to ordered is shown below:
As we know that
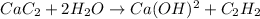
Now as we know that
1 mole of
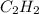 generated from 1 mole of
generated from 1 mole of
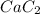
Now
26g of
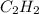 generated from 64g of
generated from 64g of
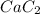
And,
26kg of
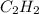 generated from
generated from
=
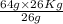
= 64kg
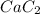
Hence, the number of kg that required for ordering the calcium carbide is 64 kg