Answer:
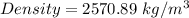
Step-by-step explanation:
Given
Mass = 6.8 g
Dimension = 1.15 cm by 1.15 cm by 2 cm
Required
Density is calculated using the following formula:
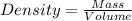
Start by converting mass from grams to kilograms;
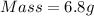
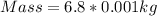
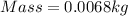
Volume is then calculated as follows;
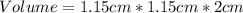
Convert cm to metres
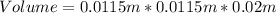
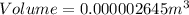
Substitute these values in the above formula;
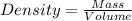
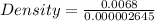
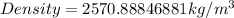
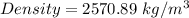 (Approximated)
(Approximated)