Answer:
1500 mmol.
Step-by-step explanation:
Hello,
In this case, for such monovalent potassium species, we can verify that 1 mEq equals 1 mmol, therefore, the required solution is shown below:
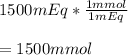
Which means that also 1500 mmol of monovalent potassium ions are contained in the liter.
Regards.