Given :
Radius of copper atom ,
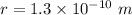 .
.
Length , l = 11.1 cm = 0.0111 m .
To Find :
How many times N can you divide to reduced to a single copper atom.
Solution :
Number of time cuts required is equal to total number of atoms .
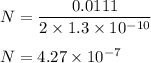
Therefore , number of time we need to divide the rod is equal to
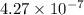 .
.
Hence , this is the required solution .