Answer:
a. dD(t)/dt = -kD(t) b.
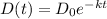 c.
c.
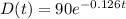 d. 5.5 hours
d. 5.5 hours
Explanation:
a. The differential equation that models the growth of the dD(t)/dt is
-dD(t)/dt ∝ D(t)
dD(t)/dt = -kD(t)
b. The general solution of the equation in a. is gotten below
dD(t)/dt = -kD(t)
separating the variables, we have
dD(t)/D(t) = -kdt
Integrating both sides, we have
∫dD(t)/D(t) = -∫kdt
㏑D(t) = -kt + c
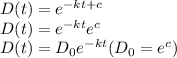
c. Given that when t = 2 hours, D(t) = 70 grams and D₀ = initial amount of drug = 90 grams
Substituting these values into the equation, we have
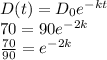
㏑(7/9) = -2k
k = -[㏑(7/9)]/2
= 0.2513/2
= 0.126
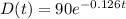
d. The half-life of the drug is the time when D(t) = D₀/2.
So
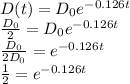
taking natural logarithm of both sides, we have
t = ㏑(1/2)/-0.126 = -0.693/-0.126 = 5.5 hours
So, the half-life is 5.5 hours